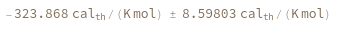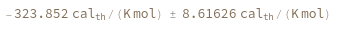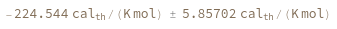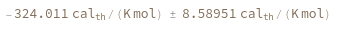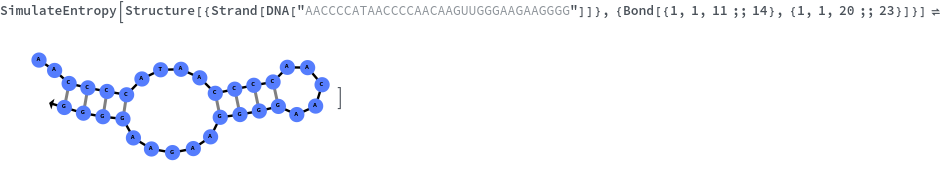SimulateEntropy
SimulateEntropy[reaction]⟹entropyObject
computes the change in entropy of the given reaction between two nucleic acid oligomers with traditional Nearest Neighbor thermodynamic analysis.
SimulateEntropy[reactantAplusB]⟹entropyObject
finds the product of reaction from 'reactantA' + 'reactantB', then computes the change in entropy.
SimulateEntropy[reactantEquilibriumProduct]⟹entropyObject
infers the type of reaction from the given 'reactant' ⇌ 'product' state and computes the entropy for that reaction.
SimulateEntropy[reactionMechanism]⟹entropyObject
computes the entropy from the reaction in the given mechanism.
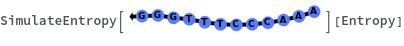
SimulateEntropy[oligomer]⟹entropyObject
considers the hybridization reaction between the given oligomer and its reverse complement.
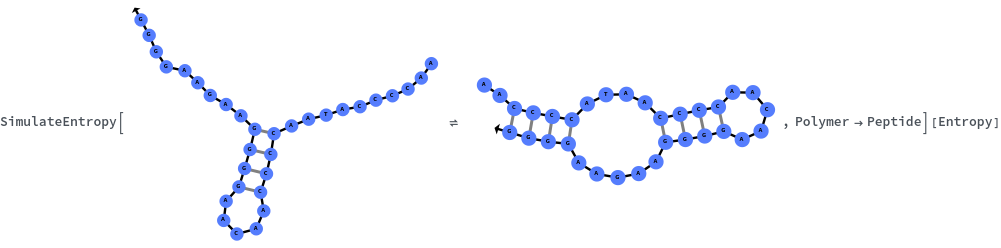
SimulateEntropy[structure]⟹entropyObject
considers the melting reaction whereby all of the bonds in the given structure are melted.
Details
- DNA Nearest Neighbor parameters from Object[Report, Literature, "id:kEJ9mqa1Jr7P"]: Allawi, Hatim T., and John SantaLucia. "Thermodynamics and NMR of internal GT mismatches in DNA." Biochemistry 36.34 (1997): 10581-10594.
- RNA Nearest Neighbor parameters from Object[Report, Literature, "id:M8n3rxYAnNkm"]: Xia, Tianbing, et al. "Thermodynamic parameters for an expanded nearest-neighbor model for formation of RNA duplexes with Watson-Crick base pairs." Biochemistry 37.42 (1998): 14719-14735.
- If given a nucleic acid sequence, strand, or sequence length, this function assumes a two-state binding between the provided sequence and a perfect reverse complement.
- Given a structure, considers only the bonded regions of the structure.
- Supported polymer types are DNA and RNA.
- Untyped sequences or lengths default to DNA if there is ambiguity.
- Enthalpy is independent of salt concentration, while entropy values for a given salt concentration. MonovalentSaltConcentration and DivalentSaltConcentration can be used to specify the concentration of monovalent salt (Na+, K+) and divalent salt (Mg2+) respectively. The entropy correction term is calculated as: 0.368*(Sequence Length - 1)*ln[(Na+) + 140*(Mg2+)] from Object[Report,Literature, "id:eGakld09nLXo"]: von Ahsen, et al. "Application of a Thermodynamic Nearest-Neighbor Model to Estimate Nucleic Acid Stability and Optimize Probe Design:Prediction of Melting Points of Multiple Mutations of Apolipoprotein B-3500 and Factor V with a Hybridization Probe Genotyping Assay on the LightCycler" Clinical Chemistry 45.12 (1999) 2094-2101.
Input
Output

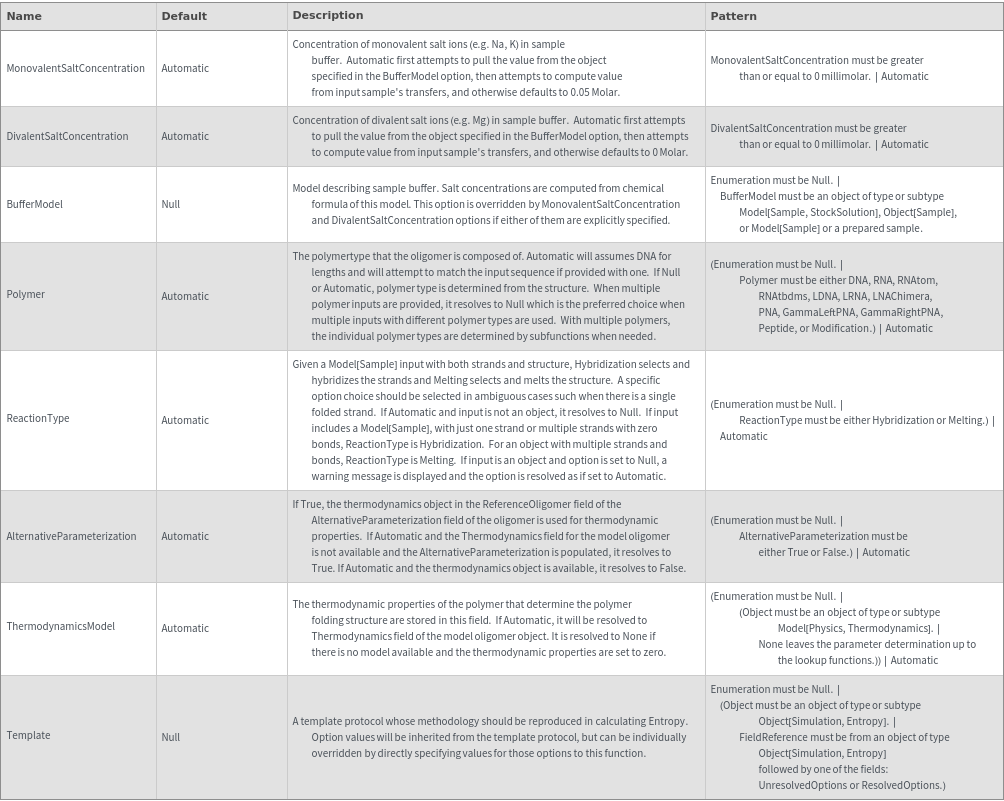
General Options
Examples
Basic Examples (5)
Compute the entropy of a hybridization reaction between given sequence and its reverse complement:


Find the product of DNA['GGACTGACGCGTTGA']+DNA['TCAACGCGTCAGTCC'], then compute the change in entropy:


Specify reaction from one structure to another:
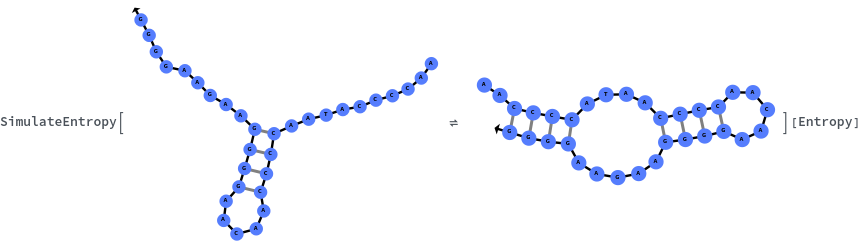

Specify reaction from one structure to another:

Compute the entropy from a simple ReactionMechanism contains only one reaction: