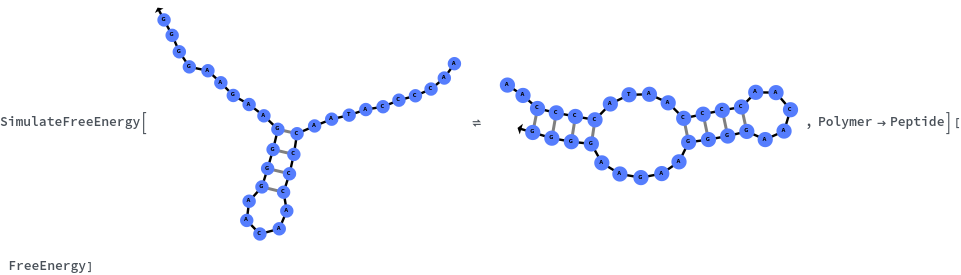
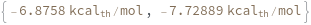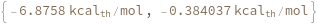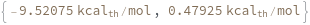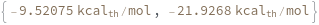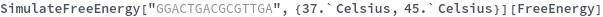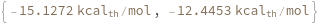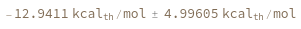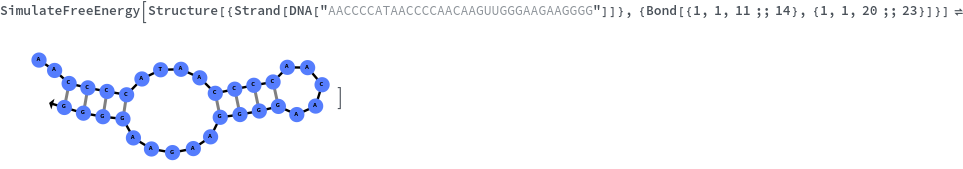SimulateFreeEnergy
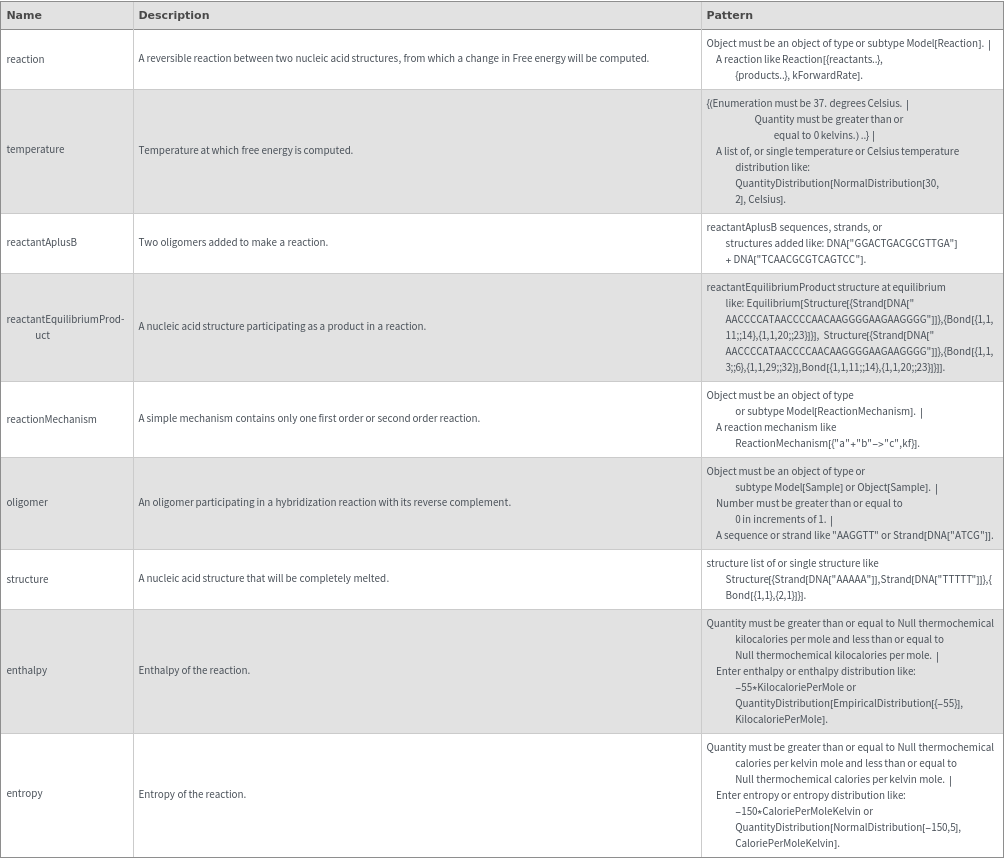
SimulateFreeEnergy[reaction, temperature]⟹freeEnergyObject
computes the free energy of the given reaction between two nucleic acid oligomers at the specified concentration with traditional Nearest Neighbor thermodynamic analysis.
SimulateFreeEnergy[reactantAplusB, temperature]⟹freeEnergyObject
finds the product of reaction from 'reactantA' + 'reactantB', then computes the free energy.
SimulateFreeEnergy[reactantEquilibriumProduct, temperature]⟹freeEnergyObject
infers the type of reaction from the given 'reactant' ⇌ 'product' state and computes the free energy for that reaction.
SimulateFreeEnergy[reactionMechanism, temperature]⟹freeEnergyObject
computes the free energy from the reaction in the given mechanism.
SimulateFreeEnergy[oligomer, temperature]⟹freeEnergyObject
considers the hybridization reaction between the given oligomer and its reverse complement.
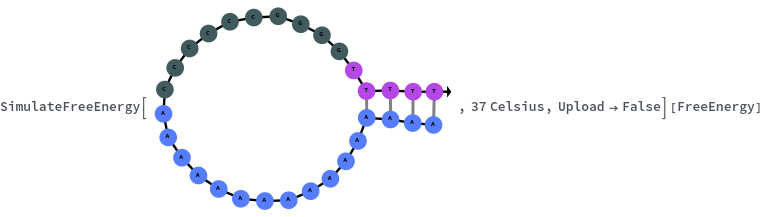
SimulateFreeEnergy[structure, temperature]⟹freeEnergyObject
considers the melting reaction whereby all of the bonds in the given structure are melted.
SimulateFreeEnergy[enthalpy, entropy, temperature]⟹freeEnergyObject
computes the free energy from the given enthalpy and entropy of a reaction.
Details
- Free energy is calculated from ΔG = ΔH - T ΔS.
- DNA Nearest Neighbor parameters from Object[Report, Literature, "id:kEJ9mqa1Jr7P"]: Allawi, Hatim T., and John SantaLucia. "Thermodynamics and NMR of internal GT mismatches in DNA." Biochemistry 36.34 (1997): 10581-10594.
- RNA Nearest Neighbor parameters from Object[Report, Literature, "id:M8n3rxYAnNkm"]: Xia, Tianbing, et al. "Thermodynamic parameters for an expanded nearest-neighbor model for formation of RNA duplexes with Watson-Crick base pairs." Biochemistry 37.42 (1998): 14719-14735.
- If given a nucleic acid sequence, strand, or sequence length, this function assumes a two-state binding between the provided sequence and a perfect reverse complement.
- Given a structure, considers only the bonded regions of the structure.
- Supported polymer types are DNA and RNA.
- Untyped sequences or lengths default to DNA if there is ambiguity.
- Enthalpy is independent of salt concentration, while entropy values for a given salt concentration. MonovalentSaltConcentration and DivalentSaltConcentration can be used to specify the concentration of monovalent salt (Na+, K+) and divalent salt (Mg2+) respectively. The entropy correction term is calculated as: 0.368*(Sequence Length - 1)*ln[(Na+) + 140*(Mg2+)] from Object[Report,Literature, "id:eGakld09nLXo"]: von Ahsen, et al. "Application of a Thermodynamic Nearest-Neighbor Model to Estimate Nucleic Acid Stability and Optimize Probe Design:Prediction of Melting Points of Multiple Mutations of Apolipoprotein B-3500 and Factor V with a Hybridization Probe Genotyping Assay on the LightCycler" Clinical Chemistry 45.12 (1999) 2094-2101.
- Temperature defaults to 37.0 °C.
Input
Output

General Options
Examples
Basic Examples (5)
Compute the Gibbs free energy of a hybridization reaction between given sequence and its reverse complement:


Find the product of DNA['GGACTGACGCGTTGA']+DNA['TCAACGCGTCAGTCC'], then compute the Gibbs free energy:


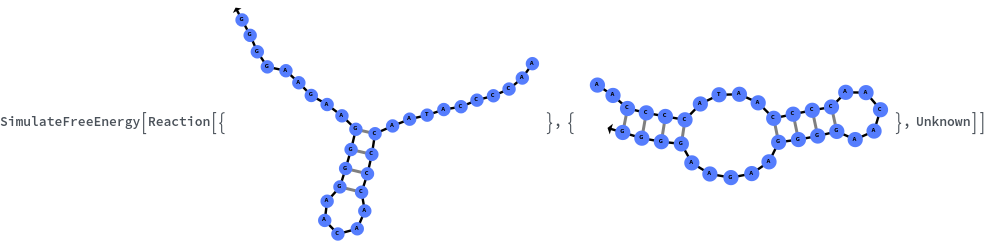
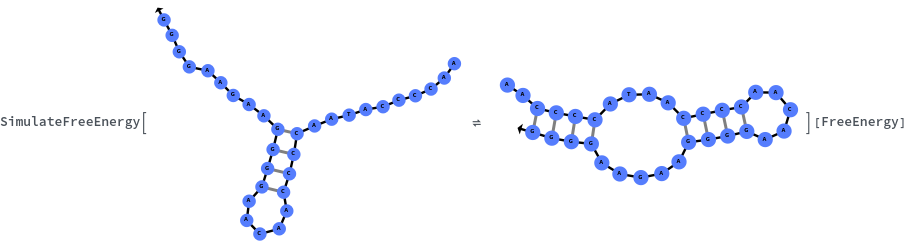
Specify reaction from one structure to another:

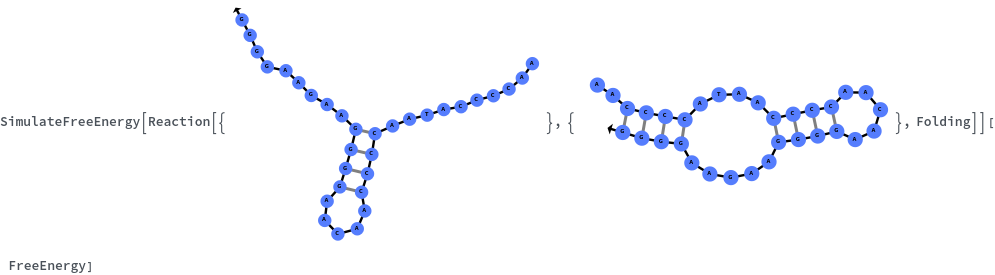
Specify reaction from one structure to another:

Compute the Gibbs free energy from entropy and enthalpy:


Additional Examples (18)
Input temperature as a distribution:
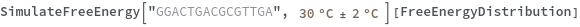
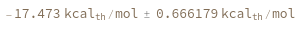
Input enthalpy, entropy and temperature as distributions:
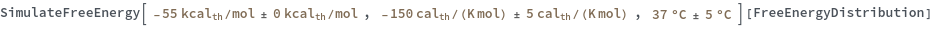
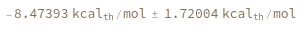
Compute the free energy from a simple ReactionMechanism contains only one reaction:


Pull strand from given sample:






Given structure, computes free energy of all bonded regions:
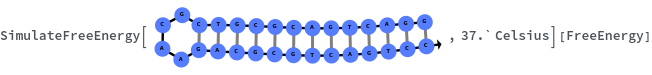

Compute the distribution of free energy of all 15-mer hybridization reactions with their reverse complements:

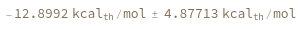


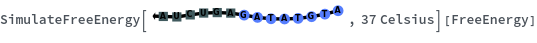

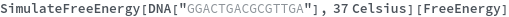


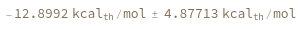
Temperature defaults to 37 Celsius:

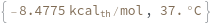
Temperature defaults to 37 Celsius:


Structure with no bonds returns zero:
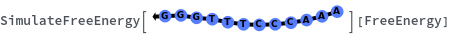

Can handle degenerate sequence and return a distribution:

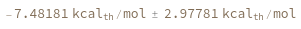
Return free energy distribution instead of mean:
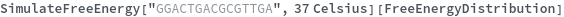

Return free energy of a structure for which the thermodynamic parameters for mismatch is not available: